Angular distribution of photoelectrons of H2
and its dependence on the internuclear distance
|
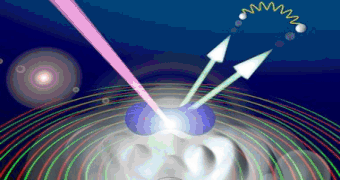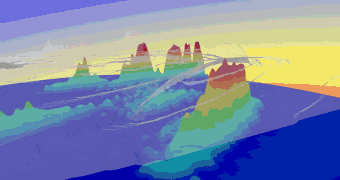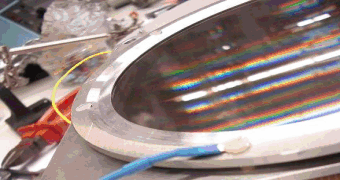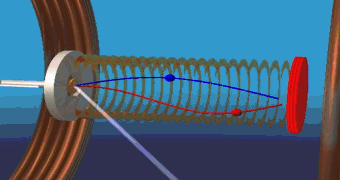
In the ground state, the internuclear distance of diatomic molecules oscillates around its mean value. The dependence of the angular distribution of photoelectrons was analyzed with Cold-Target-Recoil-Ion-Momentum-Spectroscopy[1] at Lawrence Berkeley National Laboratory. Linearly and circularly polarized 150eV-photons were used to double ionize hydrogen molecules: It is important to look at the distribution of the sum of the momenta of both electrons. Figure 1 presents a typical interference picture detected with (right) circularly polarized photons (see [2] for more information). 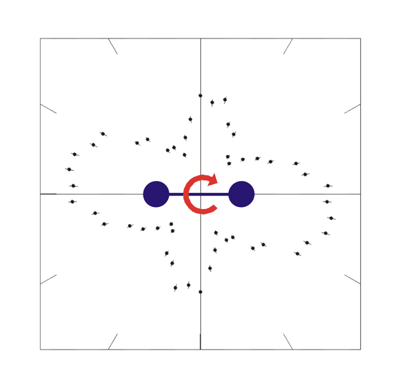
Fig.1: Photo double ionization of H2 with right circularly polarized photons: angular distribution of the sum of the momenta of both photo electrons (polarization vector of the photons in red, molecular axis in blue). After having emitted both electrons, the molecule fragments into two positively charged protons. The internuclear distance during the absorption of the photon is antiproportional to the kinetic energy of the remaining H+-ions, namely the KER[3]. Figure 2 indicates that both electrons prefer leaving the molecule perpendicular at small and parallel to its axis at larger proton distances. 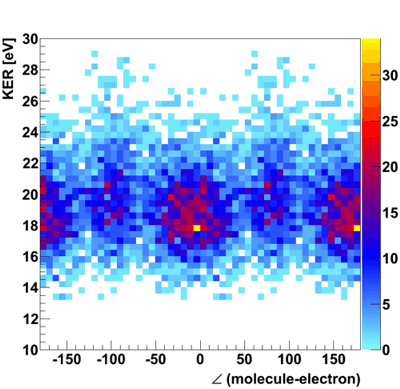 Fig.2: Photo double ionization of H2 with right circularly polarized photons: angular distribution of the sum of the electron momenta depending on the kinetic energy release (KER). To look a bit closer at this dependence, the experiment was performed with linearly polarized photons, too. The photoelectrons are then only able to leave the molecule parallel but never perpendicular to the polarization vector of the electric field[4]. By adjusting the light vector of the photons parallel or orthogonal to the molecular axis, one can cut out the horizontal or vertical maxima of figure1: 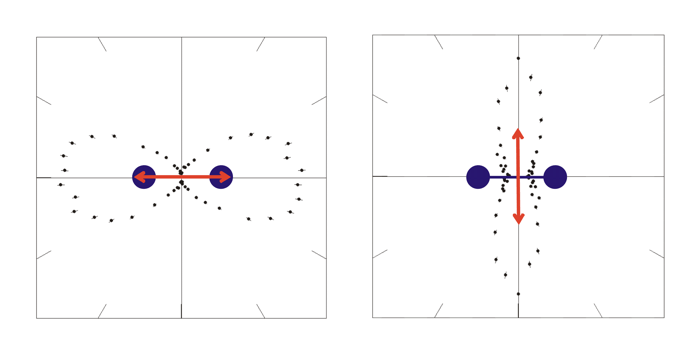 Fig.3: Photo double ionization of H2 with linearly polarized photons: angular distribution of the sum of the momenta of both electrons (left: horizontally polarized, right: vertically polarized photons; polarization vector of the photons in red, molecular axis in blue). The mean value of the KER for circularly polarized photons is 19.19eV, for horizontally polarized 18.76eV and vertically polarized photons 19.60eV. Figure 3 emphasizes this significant difference. 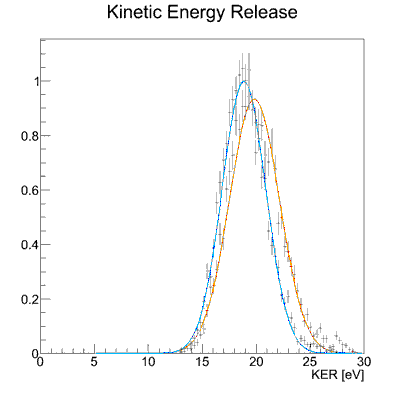 Fig.4: KER of H2 with gaussian fit (blue: horizontally polarized, red: vertically polarized photons).
References: |