|
Ion beams are a powerful tool to study atoms and molecules in the gas phase. We use them to induce dynamics within time scales from the regime of a few femto seconds down to zepto seconds, tunable by the ion velocity.
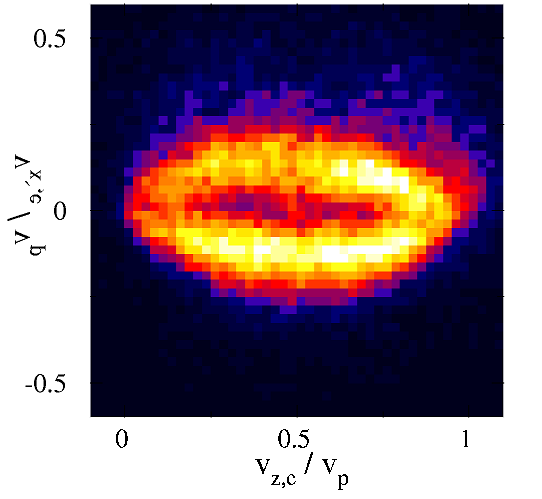 |
Slow Collisions
In slow collisions, when the speed of the ion is small with respect to the electron velocity in the orbital under investigation a transient molecule is formed and broken in the collision and the evolution of the electron cloud can be watched.
|
| |
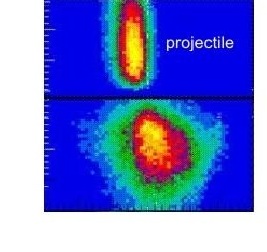 |
Fast Collisions
At intermediate velocities, when the ion is a few times faster than the electrons it can be used to rip off electrons in a capture reaction or to knock off electrons in a few body collosion.
|
| |
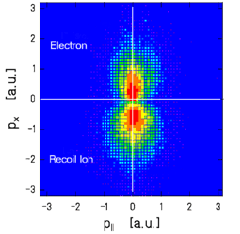 |
Relativistic Collisions
At very high velocities the ion induces a half cycle pulse shorter than an attosecond. The equivalent light field seen by the target reaches easily 1019W/cm2 and virtual photon energies up to keV. |
| |
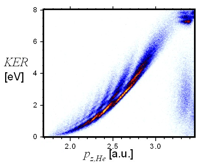 |
Dissociative Collisioins
The fragmentation of molecules following the collision with atoms allows investigating effects related to the spatial arrangement of the molecular constituents. |
|
|



