Electron diffraction self imaging of molecular fragmentation in two step double ionization of water
|
We doubly ionize H2O by single photon absorption at 43 eV leading to H+ + OH+. A direct double ionization and a sequential process in which single ionization is followed by rapid dissociation into a proton and an autoionizing OH- are identified. We obtain information about the internuclear distance at which the decay of the OH- occurs from the angular distribution of the autoionization electron. 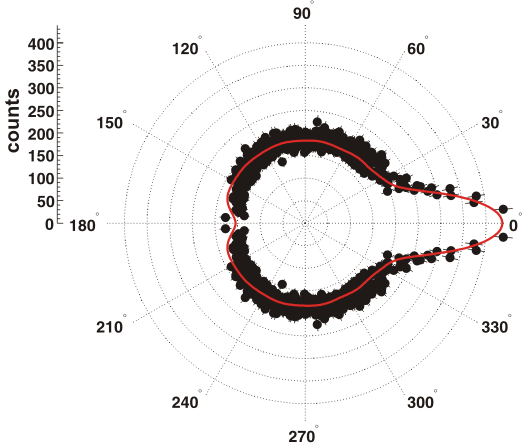 The autoionization electron shows a preferred emission in the direction of the proton. This anisotropy can be reproduced almost exactly with a simple classical scattering simulation where one Coulomb potential is located at origin and another one at a distance R simulating the proton. The electrons are launched radially from a sphere of 1 a.u. around the origin. Using R as a fitting parameter we obtain an internuclear distance of 800 a.u. at which the autoionization occurs. This corresponds to a decay time of approximately 2 ps. |
