printed page: www.atom.uni-frankfurt.de/research/20_synchrotron/30_molecules/30_dichroism
Circular dichroism in K-shell ionization of fixed in space CO and N2 molecules
|
We have measured the angular distributions of 1s-photoelectrons
excited by circularly and
linearly polarized light from fixed-in-space CO and N2 molecules, in the vicinity of
their shape resonances. A strong circular dichroism, i.e. a strong dependence on the sense of rotation of the polarization
vector of the photons, is found for both molecules.
State-of-the-art one-electron multiple scattering
and partially-correlated random phase approximation calculations are in good agreement
with many, but not all, aspects of the experimental data.
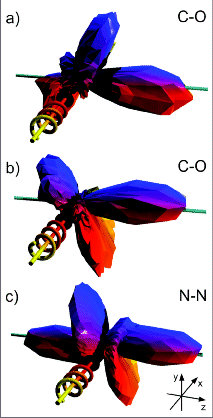
(a) and (b) - Angular distributions of C(1s)
photo-electrons (10 eV kinetic energy, on shape resonance) emitted
from a CO molecule by absorption of left and right circularly
polarized photons. The sense of rotation of the polarization
vector is indicated by the spiral, where the photon propagation vector lies along the
+ x-axis (i.e., into the page) in all cases. The molecule is aligned along the z-axis,
with the carbon atom at negative z in panels (a) and (b).
Each vertex of the three-dimensional shape represents one data point. The data have
not been smoothed, with the maximum corresponding to about 1000
counts.
(c) Analogous distribution of N(1s) photoelectrons (9
eV, on resonance) from N2.
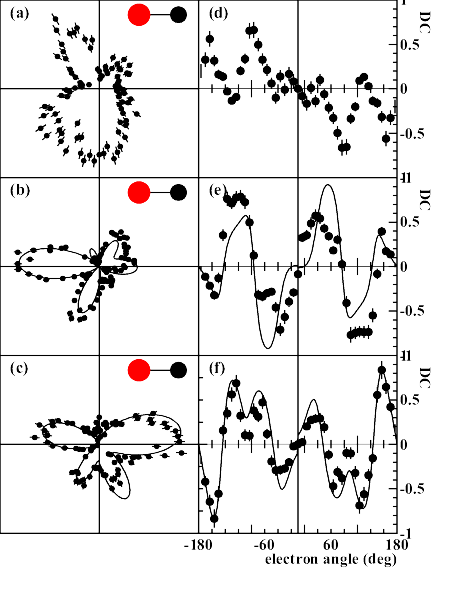
(a-c) Angular distribution of C(1s) photo electrons
emitted from a CO molecule by absorption of right circularly
polarized photons where the propagation vector of the light is
into the page. The molecule lies along the horizontal axis as
indicated, and both electrons and molecules lie within 10 degrees
of the plane of the page.
The electron energies are (a)1.6, (b)
10.0 and (c) 24.6 eV. Panels (d-f) show the corresponding circular
dichroism. Electron angle 0 corresponds to
the direction of the carbon. Full lines: Theoretical multiple
scattering calculations for the two higher energies, convoluted
with the experimental resolution.
|
|


